|
|
|
|
:
CZAPLEWSKI |
|
RESULTS AND DISCUSSION
T Predominant Orientation of Bones
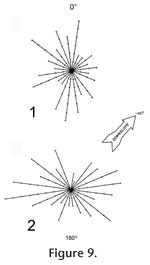
The fossils tend to lie along a diameter line with a mean angle of 171°, and the bones show no predominant directional orientation (Figure 9.1; Rayleigh's z = 1.368, p > 0.05).
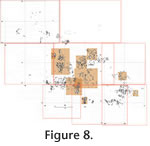
The weight of overlying sediments or diagenetic compaction appears to have flattened and crushed the fossils in situ once they were deposited. Many of the long bones, dentaries, skulls, etc., were crushed into a number of fragments, yet the various pieces retain their positions relative to each other so that the overall shape of the original element is preserved or at least can be readily visualized. For example, skulls of Prodipodomys (Figure 8.2.1, 8.2.2, 8.3.1), Geomys (Figure 8.2.3), and Sigmodon (Figure 8.2.4) are broken into many pieces but the fragments have not been dispersed by water transport. Undoubtedly, the cracking and fragmentation occurred after burial, because exposure even to light rainfall, wind, or wave action would have scattered the fragments. In fact, for the Sigmodon skull just mentioned, both dentaries remain in nearly articulated positions, and a humerus and ulna are associated (Figure 8.2.4). Virtually all but the smallest and relatively densest bones (unguals and other short phalanges, some metapodials) and teeth are crushed in this manner. All bones were crushed in a vertical direction perpendicular to the bedding plane on which the fossils were deposited. For purposes of comparison and quantification, the crushed and splintered fossil bones were considered to be "complete" as long as no significant portion was missing. Unlike the bones in the fossil bed, bones from the modern owl pellets at Sugarloaf Mountain did not exhibit a uniform circular distribution (Figure 9.2; modified Rayleigh test, u = 2.455; 0.005 < p < 0.01; Zar 1984). Mean angle was 87°, but this angle is aligned neither parallel nor perpendicular to the downslope direction (approximately 40°). The hydraulic properties of microvertebrate bones have been studied in flowing water (Dodson 1973; Korth 1979), in which they are highly susceptible to transport for considerable distances. Different skeletal elements have different "competent velocities" that are required to transport them depending on the hydrodynamic properties of the element. Below their competent velocities many bones orient themselves to a current in positions of minimum resistance (Dodson 1973). The hydrodynamic behavior of microvertebrate bones on dry land that are subjected to periodic rainfall, or in shallow standing water that are subjected to gentle wave action, has not been studied. On dry land, their orientation could be changed radically in three-dimensional space by the impact of a single drop of rain. Relative to the microtopography of the ground surface and plants growing in it, small long bones might stand on end propped up in vegetation as readily as to lie flat on the ground. If these standing bones became buried and fossilized, they might remain uptilted or laid flat depending on energy in the process burying them, and could also become flattened through compaction by the overlying sediments. Because of the lack of actualistic data concerning the hydrodynamic behavior of microvertebrate bones on dry land, the importance of the significant orientation trend of bones in the modern owl deposit remains unknown. Microvertebrate Taxa RepresentationAt least 16 taxa of vertebrates occur in the House Mountain assemblage, including a salamander (Urodela), a small finch (Passeriformes), a shrew (Soricidae), a bat (Lasiurus), and 11 kinds of rodents (Table 1). The House Mountain bone accumulation is remarkable for the relatively large number of small-bodied species represented in such a small sample area. Body-size range varies from an estimated 5 to 7 grams (shrew and salamander) to 154 to 177 grams (woodrat; Martin 1984). The combination of the small sample area, limited body-size range, and high taxonomic diversity implies that some selective factor, such as a vertebrate predator, accumulated these bones. Potential Collectors of Microvertebrate BonesThe nature of the bone assemblage suggests that a small terrestrial vertebrate predator accumulated the bones. Potential candidates are medium-sized reptilian carnivores, small to medium-sized mammalian carnivores, diurnal or nocturnal raptors, or other large predatory or carrion-feeding birds such as gulls and terns (Laridae), egrets and herons (Ardeidae), and crows and jays (Corvidae).
Many kinds of birds produce pellets of undigestible food materials (Leahy 1982; Elbroch et al. 2001). Among these, raptors (owls, hawks, eagles, vultures) are well known to produce pellets containing the remains of small vertebrates. Gulls (Laridae), herons and egrets (Ardeidae), and ravens and crows (Corvidae) occasionally eat mammalian prey, but usually not to the extent that they produce large accumulations of pellets containing bones. Furthermore, ardeids have powerful digestive systems that are capable of eliminating practically all bones when digesting prey (Glue 1970). If the House Mountain fossils actually represent prey remains, then another look at the list of taxa is instructive. Based on modern analogues, all organisms in the fossil assemblage are primarily nocturnal except for the finch or sparrow. This pattern suggests a nocturnal agent of accumulation, such as mammalian carnivores or nocturnal raptors (owls), and helps to eliminate diurnal raptors (hawks, falcons). Some extant owl species (e.g., hawk owl, Surnia ulula) often forage by day. Other owls are crepuscular or primarily nocturnal. Even nocturnal owls occasionally forage in the daytime (Vaughan 1954); barn owls and long-eared owls often raid communal roosts of small birds during winter (Glue 1972; Fritzell and Thorne 1984). This habit can account for the presence of diurnal organisms like songbirds in their diets. James (1963) believed that nocturnality of microvertebrates in the Cuyama badlands of California, USA, suggested that an owl accumulated the Cuyama fauna, and Worthy and Holdaway (1994) found mostly nocturnal ground-dwelling birds among other small vertebrates in a Quaternary bone deposit in New Zealand, where the only native mammals are bats. Perhaps the strongest evidence for a specific kind of predator is in the condition and association/dissociation of bones in the House Mountain assemblage at MNA loc. 318. First, all elements of the skeleton of prey animals, including elements not often recovered in the fossil record, are represented. Second, except for the post-burial fracturing of some elements, the bones are intact including relatively tiny and fragile ones. Third, partial association of skeletal elements originating from individual animals is frequently seen. These three points further help to eliminate diurnal raptors and eliminate mammalian carnivores as the predators responsible for the Verde assemblage, because diurnal raptors and mammalian carnivores typically chew and break up the bones of their prey (Glue 1970; Mayhew 1977; Korth 1979; Andrews and Nesbit Evans 1983). Diurnal raptors also cause considerably more corrosion to bones than owls because their gastric juices are stronger (Duke et al. 1975). Owls typically do little physical or chemical damage to prey bones (Glue 1970; Mayhew 1977; Dodson and Wexlar 1979). Owls often produce one pellet per prey item (but this varies; see below) and, given the rate at which they hunt and kill prey, they can produce several pellets in a day. During cyclic irruptions in high-latitude populations of arvicoline rodents (voles and lemmings), some owls can gather large numbers of prey very quickly (Hanski et al. 1991, 2001; Klemola et al. 2002; Korpimäki and Norrdahl 1991; Korpimäki et al. 2004). The pellets are usually cast beneath the daytime roost or nest, where large numbers of pellets can accumulate. Sometimes owls cast pellets in their nocturnal hunting grounds, and the pellets may be concentrated at habitual night roosts or scattered. The size and shape of pellets roughly reflect body size and internal anatomy. These characteristics as well as the condition of the contents can be helpful in identifying the producer of modern pellets (Elbroch et al. 2001). When owls eat, their prey is swallowed whole or torn into chunks, which is then swallowed with varying degrees of bone breakage (depending on the species of owl; Elbroch et al. 2001). Large prey may be partly consumed and the uneaten portions cached for later consumption (Forsman et al. 1984). In the proventriculus, flesh and skin are digested but bones, fur, or feathers are usually little corroded and are formed into a pellet that is then spewed onto the ground (Duke et al. 1976; Duke and Rhoades 1977; Rhoades and Duke 1977). Regurgitated owl pellets often exhibit association of elements from individual prey skeletons unless prey was torn up in order to be swallowed; the degree of breakage also varies between and within owl species (Dodson and Wexlar 1979; Hoffman 1988). Lyman et al. (2003) noted that, as the size of the most abundant mammalian prey taxon in a barn owl pellet decreases, the mean number of individuals per pellet increases, and that larger barn owl pellets contain more individual prey than smaller pellets. If the pellets lie on open ground for a time, they can be disintegrated by rainfall, wind, and/or by the activities of scavenging insects and other invertebrates, so that the associated bones they contain become dissociated and scattered (Terry 2004). Weather and associated moisture strongly affect the speed with which an owl pellet breaks down, and the enclosed bones become separated (Elbroch et al. 2001). Many of the bones in the modern owl pellet accumulation I examined at Sugarloaf Mountain were also trampled and broken by cattle.
When mammalian carnivores and diurnal raptors eat vertebrate prey, the prey's bones are always broken to a mu By comparison, in about one liter of the modern barn owl pellets collected near Rodeo, New Mexico, one baculum and one malleus of Dipodomys spectabilis were found among the remains of a minimum of three individuals of that species (based on number of skulls, auditory bullae, and dentaries); one baculum and one malleus of Dipodomys merriami were recovered among remains of a minimum of nine individuals of that species (based on cranial elements). In addition, at Rodeo, 1 baculum, but no auditory ossicles, were found per four Chaetodipus hispidus skulls, and one baculum was found per 26 Perognathus flavus right dentaries. No other bacula or auditory ossicles were recovered, even though skeletal elements of a pocket gopher and seven genera of cricetid rodents (among other taxa) occurred. In modern great horned owl pellets collected near Camp Verde, Arizona, two bacula and one malleus were found per 18 Dipodomys ordii crania; one baculum per 17 Peromyscus dentaries; one incus per 7 Thomomys bottae crania; and one cricetid incus fragment of indeterminate genus from among a total of 47 individuals of Reithrodontomys, Peromyscus, and Onychomys. No bacula or auditory ossicles of Perognathus or Neotoma were recovered per one and 22 skulls, respectively. Although most of the House Mountain fossils are isolated bones (disarticulated and dissociated), many examples can be found which represent partial association of elements or partial skeletons from individual animals. In the master taphonomic map (Figure 8.1.1, 8.1.2, 8.1.3, 8.1.4, 8.1.5, 8.1.6, 8.1.7, 8.1.8), much of the west half of grid square 2N+1W is occupied by a slightly dissociated anterior appendicular skeleton and skull of a pocket gopher, Geomys (Nerterogeomys) minor (Figure 8.2.5). In this area there is no redundancy of elements, indicating that the anterior half of a single prey animal is preserved. Additional examples of partial association are evident: In the southwest corner of grid square 2N+00 and northeast half of adjacent grid square 2N+1W are a right and left humerus (Figure 8.2.6), a scapular fragment, right tibiotarsal fragment, right coracoid, right ulna, and premaxilla of a small passeriform bird (Emberizidae or Fringillidae). In the southwest corner of grid square 3N+2W is a crushed skull of Sigmodon mentioned previously (Figure 8.2.4). It retains both dentaries in articulation and an associated humerus and ulna. In the southeastern corner of grid square 1S+3W is a skull of Prodipodomys (Figure. 8.2.1). The well-preserved cranium is associated with a right dentary bearing the incisor and two cheek teeth, and two loose left cheek teeth with tooth wear equivalent to teeth in the cranium. The partial association of fossil bones in the House Mountain assemblage probably reflects burial after a period of exposure to weathering long enough to cause partial pellet disintegration. Modern owl pellet accumulations in unprotected sites normally include pellets in various stages of disintegration, ranging from freshly cast, intact pellets to completely disintegrated pellets (Figure 5.2). Age-Frequency of PreyPrey individuals of different ontogenetic ages are differentially vulnerable to predation by owls (Lay 1974; Longland and Jenkins 1987). Nevertheless, age-frequency profiles are sometimes used to indicate whether a fossil assemblage was the result of catastrophic or attritional death (Voorhies 1969; Shipman 1981; Klein 1982; Korth and Evander 1986; Kos 2003; McFarlane and Blake 2005). Catastrophic death assemblages are those that result from a relatively instantaneous killing that is not age-selective, and all members of a species are likely to be killed equally. An attritional death assemblage is one that is the result of selective death of the most vulnerable individuals of a population (very young and very old).
Owl Pellets, Owl Diets, and Microvertebrate Fossil AccumulationsAs noted above, owl pellets probably are the source of microvertebrate assemblages in many previous studies, especially Pleistocene sites in caves or sinkholes (Mayhew 1977; Walton 1990), although some assemblages may represent scats, pellets, or caches made by other carnivores (Mellett 1974; Mead and Van Devender 1981; Andrews and Nesbit Evans 1983). Owls have a fossil record dating back to the Paleocene, and at least four genera are known in the Pliocene of North America (Brodkorb 1971; Rich and Bohaska 1976, 1981; Walton 1990). Extant owls, Strigidae and Tytonidae collectively, inhabit all land surfaces of the world except for a few oceanic islands and Antarctica. In fact the barn owl, Tyto alba, is one of the most widespread of all land bird species (Van Tyne and Berger 1976; Marti et al. 2005). Studies of owl diets using regurgitation pellets have been nearly as widespread as owls themselves (e.g., Cowan 1942; Hawbecker 1945; Anderson and Long 1961; Glue 1967, 1970; Haverschmidt 1970; Buden 1974; Marti 1974; Czaplewski 1976; Herrera and Hiraldo 1976; Mayhew 1977; Thomas and Thomas 1977; Jaksíc and Yañez 1979; Dickerman and Brash 1980; Herrera and Jaksíc 1980; Justo and DeSantis 1982; DeSantis et al. 1983; Baker 1986; Taberlet and Fumagalli 1996; Fernandez-Jalvo et al. 1999; Bilney et al. 2006; Goodman and Griffiths 2006) and are routinely conducted in high school and elementary school classes as basic ecology lessons. Undoubtedly, their wide range and their habit of forming pellets of well-preserved bones have resulted in owls being major contributors to the global fossil record of small vertebrates. In a study of modern owl pellets decomposing on a forest floor, Terry (2004) noted that as owl pellets disintegrate the skeletal composition changes from a high proportion of small and fragile elements to a high proportion of larger, more robust skeletal elements. She concluded that the small, fragile bones initially are protected within the matted hair of the regurgitated pellet but, as they become exposed by physical and chemical weathering on the ground, their previous etching and pitting in the owl's digestive tract makes them more prone to fragmentation and disappearance than the larger bones. Dodson and Wexlar (1979) calculated the proportional representation of skeletal elements in pellet-derived bone concentrations from pellets of captive owls. I tried to repeat their methods with field data for modern and fossil owl pellets, and found a greater loss of information under the less ideal and older circumstances in the fossil deposit. A larger proportion of the bones were broken, possibly due to trampling by large hoofed mammals (although there is yet no evidence of this in the fossil record), or weathering, or perhaps greater breakage by the owls. In the House Mountain assemblage, post-burial crushing has rendered species identification of most postcranial elements difficult or impossible. The breakage also made it difficult to accurately estimate the actual number of bones present. Instead of a count of complete (intact) elements, I counted a number of identifiable specimens (NISP), fragmented and intact. As a basis for comparison, I counted the minimum number of individual (MNI) rodents indicated by the most numerous element, maxillae, dentaries, or cheek teeth. The percentage present (percent representation) expresses the proportion of individual specimens present relative to the potential or expected number of intact bones (NIb) per rodent skeleton (Table 5). Very few elements in the House Mountain fossil accumulation were complete due to post-depositional flattening and breakage. As a result, in both the disintegrated modern pellets and the microvertebrate fossils, the NISP often is excessive relative to the potential Nib because of multiple fragmentation of a given element (Table 3 gives percentages of intact quarried elements at House Mountain). Thus, a single incisor broken four times would give an NISP = 5, overestimating the expected NIb by 500 percent.
Body Size of the Hypothetical Owl Implicated in the House Mountain Assemblage
Surprisingly, the remains of rabbits are lacking in the House Mountain assemblage, which contrasts with their occurrence at most other vertebrate fossil localities in the Verde Formation. Modern owls large enough to eat rabbits do so regularly (Earhart and Johnson 1970) and even 150 g burrowing owls were recorded to eat cottontails (Marti 1974). By analogy with extant owls, the presence of only adults of the largest prey species (Neotoma vaughani) suggests that the owl(s) could have preyed on juveniles of larger species (i.e., rabbits). In a Pleistocene agglomeration of bones from owl pellets in Argentina,
Tonni and Fidalgo (1983) found mostly the remains of juveniles of the larger micromammals associated with adults of smaller micromammals. Young rabbit remains may possibly yet be found in the
Earhart and Johnson (1970) provided a summary of food habits and body masses of several North American owls. The body mass of the largest prey species increases with the body mass of the owl (Figure 14). Using Martin's (1984) equation for the relationship between first lower molar length and body mass in cricetine rodents, Pliocene woodrats from House Mountain weighed between 154 and 177 g. If this body mass is eye-fitted into the trend in Figure 14, it gives an approximation of the body mass of the owl between 100 and 400 g. House Mountain Microvertebrates and PaleoecologyIn the House Mountain assemblage, the microvertebrate taxa broadly reflect the putative Pliocene wetlands, grasslands, and open savanna suggested for the Verde Formation by earlier authors (Nations et al. 1981; Table 6). Based on habitat-selection similarities with phylogenetically related taxa, the unidentified shrew and red bat (Lasiurus cf. L. blossevillii; Czaplewski 1987b) could have utilized wetlands and riparian gallery forest, as well as "edge" habitats transitional between forest and grassland. Sigmodon spp. are grazing rodents common in grasslands and oak-savanna-grasslands of southwestern North America today, and the fossil species and Prosigmodon likely were also. The other small vertebrate taxa do not conflict with the other hypothesized paleocommunity types at MNA loc. 318. However, pollen and sedimentologic data from this locality provide better sources of vegetational information (Nations et al. 1981) for this portion of the Verde Formation. For example, pollen analysis of the Verde Formation sediments by Hevly (in Nations et al. 1981) previously revealed the presence of deciduous gallery forest along the lake edge and inflowing streams—possibly owl pellets were cast from a roost in a lakeside sycamore, cottonwood, or other tree. More detailed information from the vertebrate paleocommunity, however, depends on the recovery of larger samples or additional equivalent-aged samples of fossils, or possibly on studies of stable isotopes in the sediments or fossils. The nearest vertebrate locality in the Verde Formation, both stratigraphically and horizontally, to MNA loc. 318 is MNA loc. 319. MNA 319 is 1.5 km from 318 (Czaplewski 1987b); paleomagnetically, MNA 319 is just below the base of the Nunivak normal polarity zone (Bressler and Butler 1978; now chron C3n.2n of Berggren et al. 1995; Bell et al. 2004), about 4.62 million years old, or roughly 300,000 years older than MNA loc. 318. The vertebrate assemblage from MNA 319 differs in some taxa from that of 318 and includes Leporidae, Prodipodomys idahoensis, Postcopemys n. sp. (Lindsay and Czaplewski, this volume), Sigmodon minor, Neotoma (Paraneotoma) vaughani, Ogmodontomys cf. poaphagus, a second indeterminate arvicoline, and Bassariscus sp. (Czaplewski 1990). Most of these taxa are rare except for Ogmodontomys. Perhaps this assemblage reflects the biases inherent in the diet of a different predator (the ringtail, Bassariscus?), as well as a different time period, but with partial overlap of prey selection and habitat selection in that Prodipodomys, Postcopemys, Sigmodon, and Neotoma are shared between the two localities. Time-AveragingThe phenomenon of time-averaging is often a consideration in taphonomic studies of vertebrate fossil accumulations. Assemblages of mammal bones by predators are generally considered to represent durations from 10-1 to 102 years (Behrensmeyer and Chapman 1993). It seems possible that suitable roosting and nesting sites could be at a premium for owls, and particularly good sites might be used for many generations. With respect to the duration of modern owl pellet accumulations, some investigators have revisited a site to collect owl pellets on occasions up to 46 years apart, which presumably was continuously occupied by the owls during that interval (Kittredge et al. 2006; Marti 1973; Baker 1986). Conceivably, owls might occupy a favorable site and accumulate prey remains for months to tens or even hundreds of years. One of the modern owl roosts sampled for this study showed a sizeable accumulation of prey-animal bones in the 1980s (Figure 5, Figure 6); however, bones were virtually absent, and this roost had been abandoned when revisited in 2007, possibly due to the construction of a resort nearby and increased human activity in the formerly remote area. Not surprisingly, the density of bones at the modern sites visited varied across the ground surface beneath the roosting cliff, along which many different perches were available. Terry (2004) showed that the density of skeletal elements derived from owl pellets decreases with the distance from the base of a roost tree. In the very small area (approximately one-fourth of a square meter) of the fossiliferous bedding plane quarried and mapped at House Mountain, the density of fossil bones varied from none in some grid squares to full coverage in other grid squares. It is impossible to know the distance from a conjectural owl roost and whether or not bone density in this area is representative of the entire (unquarried and unscreenwashed) microvertebrate deposit. However, the available evidence indicates that the deposit represents a time-averaged duration within the same range (months to hundreds of years) as other predator-accumulated assemblages noted by Behrensmeyer and Chapman (1993). Because the fossiliferous deposit is virtually restricted to one thin bedding plane, with very few microvertebrate bones found by screenwashing the strata immediately above and below it, the deposit might represent a relatively short-term (10-1 to 101 years) accumulation of bones followed by sedimentary burial of the bones and pellets and abandonment of the local roost by the owl. |
|