|
|
|
|
:
CZAPLEWSKI |
|
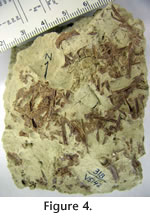
METHODS AND MATERIALSQuarried Blocks with Microvertebrate FossilsMNA locality (loc.) 318 (Figure 3.1) occurs in a 2 m high exposure (8 m in lateral extent) of calcareous grayish-green mudrocks interbedded between white biogenic limestones (micrites) containing occasional external molds of freshwater gastropods. Screenwashing of the mudrocks from the locality and inspection in the field had previously indicated that abundant microvertebrate fossils and rare gastropod fragments were exposed on edge in a single bedding plane for about 1 m along the face of the outcrop (Figure 3.2). Screened samples from above this bedding plane had produced no small vertebrate remains, and samples screened below the bedding plane produced very few. Therefore, the fossiliferous horizon in the cliff face was quarried by blocks.
Taphonomic data collection followed Munthe and McLeod (1975), and data analyses followed approaches much like those of Maas (1985) and Dodson and Wexlar (1979), along with the application of biological as well as taphonomic criteria (e.g., Korth and Evander 1986). The trend (compass orientation) of the proximal end of 82 identifiable, elongate bones was measured, and their frequencies among 24 sectors of 15° each was plotted on a circular histogram. If proximal and distal ends were broken off or indiscernible, the azimuth of the larger end was measured. If there was no difference in size between ends, the bone was not used in the analysis. Plunge was not measured because all the bones were lying essentially horizontal on a relatively flat bedding plane. Nevertheless, the circular distribution data were treated as a diametrically bimodal distribution (Zar 1984) in case no significant direction of orientation occurred and a mean angle of orientation could still be calculated. The nonparametric Rayleigh test (Zar 1984) was used to test the null hypothesis that the sampled population of bones is uniformly (randomly) distributed. An attempt was made to identify all skeletal elements, and where possible, to identify the species to which certain skeletal elements belonged. Some teeth, dentaries, and skull fragments were carefully removed from the prepared quarried blocks after mapping. This procedure was necessary to identify small mammals whose tooth crowns were either buried in the sediment or lying obliquely, thereby preventing detailed in situ evaluation with a microscope. The removed elements were assigned numbers that were recorded on individual block maps. These fossils were then completely prepared, usually with the aid of water-soluble Carbo-Wax (General Electric Co.). All fossils were examined for signs of abrasion and transport by water. In quantifying the vertebrate taxa at the House Mountain fossil locality, the relative abundance was based on crania, dentaries, and isolated premolars and molars (Table 1). No attempt was made to count symmetrical pairs of paired elements or greatest number of ipsilateral elements. Nor was any attempt made to match or to separate pairs of contralateral elements based on similar degrees of tooth wear. A simple total number of halves for each paired element was counted and divided by two in order to determine the MNI represented. Thus, Table 1 may slightly underestimate the actual numbers present in the deposit. Modern Owl Pellets for Comparison
Recent owl pellets were collected from several localities in Arizona in order to make taphonomic comparisons with the fossil assemblage from House Mountain. Owl pellets analyzed in the greatest detail came from three localities. The first two localities, occupied by the barn owl (Tyto alba), occurred in agriculturalized mesquite grassland between Portal, Arizona, and Rodeo, New Mexico. These materials were analyzed by generally following the methods of
Dodson
and Wexlar (1979) on pellets gathered from captive owls. Counts were made of each skeletal element except ribs, and the degree of completeness and breakage patterns of specific elements were noted. Mammal, bird, reptile, and amphibian skeletal elements were tallied separately. No species identifications were made for postcranial bones of mammals from these sites, but cranial elements were identified at lower taxonomic levels. A count of the minimum number of individuals (MNI) was based on major elements (e.g., dentary, femur). Data from the modern owl pellets, including prey taxa represented, proportional representation of skeletal elements, and patterns of bone breakage were compared with published studies and with the fossils from House Mountain.
The third modern pellet locality was a habitual roost for barn owls at a short cliff south of Sugarloaf Mountain, Yavapai County, Arizona. Along the base of this cliff was an accumulation of small vertebrate bones from regurgitation pellets (Figure 5.1-5.4) representing prey remains from many seasons. The exact duration of accumulation is unknown, but probably represents at least one season of occupation of the roost.
A few modern pellets were collected opportunistically near Camp Verde, Arizona, from a great horned owl (Bubo virginianus) roost. These pellets were examined in less detail than were the barn owl pellets from the other localities, but some incidental results are mentioned below.
|
|